Education

Search
Saved Activities
0 Activities
0 Activities
Filter Content
Recommend to a friend
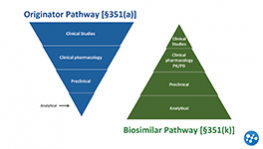
FDA Criteria: Biosimilar Approval, Manufacturing, & Naming
Upon completion of the activity, participants should be able to explain the data package required by the FDA for biosimilar approval and explain how this differs from a biologic ‘standalone’ development program.

A ACCME Accredited provider.
The France Foundation ©
Chandler Building, 84 Lyme Street, Old Lyme, CT USA