Webinars

Search
Saved Activities
0 Activities
0 Activities
Filter Content

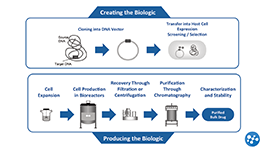
What is a Biologic?
Array
Activity Info
Upon completion of the activity, participants should be able to:1. Answer patient questions about the differences between biologics and small-molecule drugs
2. Explain the FDA process for the evaluation of post approval changes for reference biologics, and how this relates to biosimilars
CME/CE credit is no longer available for this activity.
Tags
Webinar | Oncology | 0.25 - 15 mins | Dermatology | Gastroenterology | Hematology | Rheumatology | Nurse | Biosimilars | BiosimilarsCME.org | Online | Pharmacist |

A ACCME Accredited provider.
The France Foundation ©
Chandler Building, 84 Lyme Street, Old Lyme, CT USA